short menu
About EUREC
National Information
Services
- Event Calendar
- Information for Researchers
- Training Materials
- Login for EUREC members

This network has received funding from the European Union.
Eurecnet - National Information: Germany
National Information: Romania
- Website of the NEC: http://cne.ancs.ro/ (Ro.)
- Page link of the NASR: http://research.ro/ro/articol/980/despre-ancs-prezentare (Eng.)
- Page link of the NAMMD: http://www.anm.ro/anmdm/en/despre_institutie.html (Eng.)
- SCD (Scientific Council Decisions) No. 39/2006- Guidelines on Good Clinical Practice (link- Ro.)
- SCD No. 40/2006 - Guidance on general considerations about clinical trials (link- Ro.)
- SCD No. 41/2006 - Guideline on clinical investigation of medicinal products in the pediatric population (link- Ro.)
- SCD No. 50/2006 - Guidance on the application forms and documentation to be sent to bioethics committee to obtain its opinion on the development of a clinical trial with human medicines (link- Ro.)
- SCD No. 22/2010 – Guideline on the application for authorization of a clinical trial with a drug for human use (link- Ro.)
- SCD No. 6/05.06.2014 - Regulations on the authorization of non-interventional studies conducted with human medicines (link- Ro.)
- SCD No. 27/2011 - Guideline on the collection, verification and reporting of events/adverse reactions occurred in clinical trials with medicines for human use (link- Ro.)
- MHO (Minister of Health Order) No. 891/2006 - Model Sheet for the report of adverse drug reactions (link- Ro.)
- MHO No. 903/2006 - Principles and detailed guidelines on good clinical practice for medicinal products for human investigation, and requirements for the manufacture and importation of such products (link- Ro.)
- MHO No. 904/2006 - Rules on the implementation of good practice in the conduct of clinical trials on medicinal products for human use (link- without English translation)
- MHO No. 906/2006 - Rules and analytical protocols on drug testing (link- Ro.)
- MHO No. 912/2006 - Regulations for the approval of the institutions who may conduct clinical trials in human medicines (link- Ro.)
- MHO No. 615/2010 on approval of No. 906/2006 (link- Ro.)
- Order no. 398 of 21 March 2013 (*updated) for the consultative commissions of the Ministry of Health (*Updated till date September 14, 2016) (link-Ro)
- Law No. 206/2004, Art. 9(1) related to the good conduct of scientific research, development, technology and innovation (link- Ro.)
- The decision No. 25/03/07/2015 - approving the amendment and completion of the Annex No.6 / 04.22.2014 regarding the approval of the Regulations on the authorization by the National Agency for Medicines and Medical Devices of clinical trials (link- Ro.)
- EFGCP. (2011). The EFGCP Report on the Procedure for the Ethical Review of Protocols for Clinical Research Projects in Europe.
- National Authority for Scientific Research and Innovation. Retrieved from Ministry of National Education and Scientific Research: http://research.ro/ro/articol/980/despre-ancs-prezentare
- National Ethics Council. Retrieved from National Ethics Council: http://cne.ancs.ro/
- National Agency for Medicines and Medical Devices. Retrieved from Ministry of Health: http://www.anm.ro/anmdm/en/despre_institutie.html
- National Committee of Medicines and Medical Devices. Retrieved from National Committee of Medicines and Medical Devices: http://en.bioetica-medicala.ro/
- Icahn School of Medicine at Mount Sinai (USA)
- Clarkson University (USA)
- Vilnius University (Lithuania)
Short description of RECs system:
(1) National Committee of Medicines and Medical Devices (NCMMD):
in Romania NCMMD is one of the main institutions responsible for the ethical review of a clinical trial or for an investigational medicinal product. Its mission is to ensure that biomedical research is conducted ethically and it intervenes in the research process (before, during and after a research is approved, and also when the research results are evaluated and reported), to protect life, dignity, health, rights, comfort and safety of a clinical trial. This committee is mainly responsible for biomedical research, including research on biological materials or studies on surgical interventions, however, other non-biomedical research can be revised upon request. For more details about NCMMD in the research process, please consult this link.NCMMD is an independent body in the coordination of the Health Minister and the President of the Academy of Medical Sciences, whose members were selected by the Minister of Health no. 34/09.01.2014, following the proposals of Academy of Medical Sciences, on the basis of the General Assembly elections.
Regarding the educational background of its members, on the web page of the committee it can be found that NCMMD includes members such as professional scientists, professionals in the health care, lawyers and people with expertise in ethics. Other relevant disciplines are required like: epidemiology, clinical pharmacology, pharmacy, psychology, sociology and biostatistics.
Related to the organizational structure of the NCMMD, Fig 1 present a chart also available on the web page of the committee:
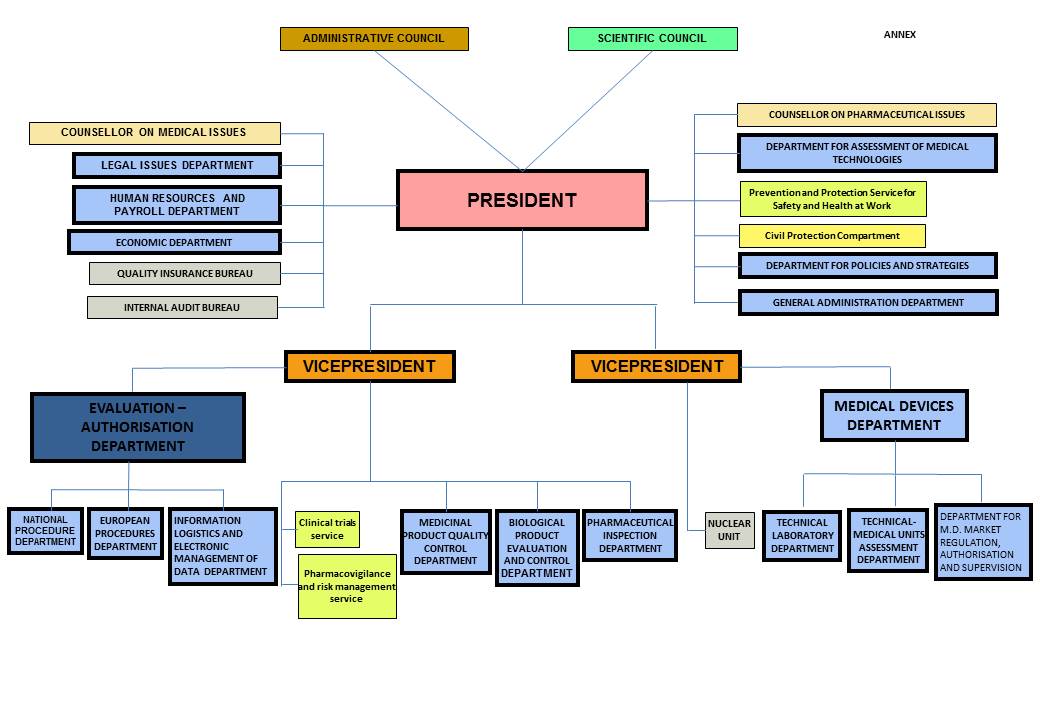
Figure 1 National Commission of Medicines and Medical Devices, OMS no. 34/2010 organization chart
Concerning the conflict of interest, according to Order no. 398 of 21 March 2013 (*updated) for the consultative commissions of the Ministry of Health (*Updated till date September 14, 2016) (link-Ro), "the members of an advisory committees are submitted to the Ministry of Health criteria, regarding the confidentiality and the conflict of interest, according to the regulations of the organization and its functioning (Article 7, (1)). This declaration has to be updated each year (Article 7, (3)).
On the webpage of the committee there is also available information related to the status of submitted and approved clinical studies. For more details, please consult this link.
Website of the NCMMD: http://en.bioetica-medicala.ro/
(2) National Agency for Medicines and Medical Devices (NAMMD):
the other main institution is NAMMD who also provides procedure steps for assessment and approval of applications for clinical trials with human medicine. This committee is mainly responsible for the revise of clinical trials on medicinal products.NAMMD is a public institution subordinated to the Ministry of Health, established through Government Emergency Ordinance no. 72 of 30 June 2010, whose first mission is to protect and promote public health. “The most important strategic objective of the NAMMD is promotion and protection of public health, by the accomplishment of the NAMMD primary role, namely warranty of compliance of authorized medicinal products with the required standards and intended purpose as well as of their acceptable level of safety”.
An organizational chart of NAMMD is illustrated below in Fig 2, as it appears on the committee web page:
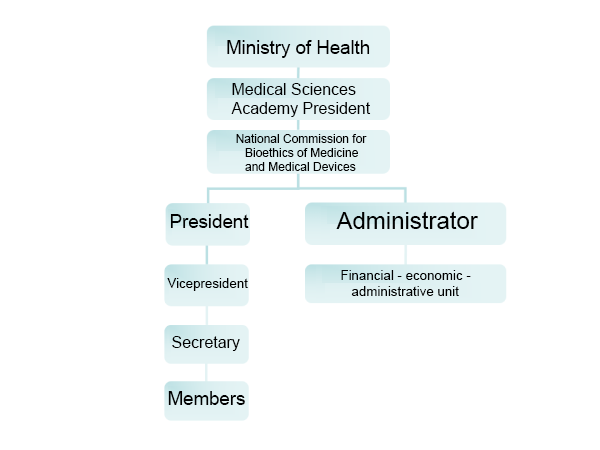
Figure 2 Organizational chart for National Agency of Medicines and Medical Devices
(3) National Ethics Council (NEC):
regarding the coordination and monitoring of the application of the norms of moral and professional conduct in research and development activities, there is also the NEC who has a consultative body status. NEC is affiliated to National Authority for Scientific Research and Innovation (NASR) who is a specialized body with legal personality in the subordination of the Ministry of National Education and Scientific Research. NASR has a leading status on the national system of scientific research, technological development and innovation; it is exercising its power by laws and other normative acts together with ministries relevant government policy.
(4) Institutional Ethics Committees (IECs):
According to the Law No. 206/2004, Art. 9 (1) related to the good conduct of scientific research, development, technology and innovation, there are also Institutional Ethics Committees who are established in those institutions who are part of the national system of research and innovation and other units who are providing the validation of the results (e.g. universities or hospitals). Their role is the fulfilling of the specific codes and the resolution of various complaints received. They have an independent body status with a consultative role regarding the safeguarding of the rights, safety and the comfort of the participants in the clinical trials.
Specific Legislation:
Medical Bioethics in EU LegislationSome legislations of Medical Bioethics from Romania:
Networking between RECs related to the biomedical research review (1), (2) - (4):
a) Concerning the ethical review of biomedical research, it is mandatory to obtain the favorable opinion of NCMMD (1) for conducting a bio-medical research (clinical drug trials – CDT; for details about the ethics approval networking for clinical research except for CDT, consult point d) from below).
b) The interaction procedure between National Committee of Medicines and Medical Devices (NCMMD) (1) and the National Agency for Medicines and Medical Devices (NAMMD) (2) is established only regarding a clinical drug trial and is stipulated in The decision No. 25/03/07/2015 - approving the amendment and completion of the Annex No.6 / 04.22.2014 regarding the approval of the Regulations on the authorization by the National Agency for Medicines and Medical Devices of clinical trials:
“a clinical trial may begin only if NAMMD authorized the clinical trial and NCMMD submitted a favorable opinion” (Unic Art., (3))
c) The Institutional Ethics Committees (IECs) (4) have only a consultative status regarding bio-medical research. Concerning non-biomedical research, the IECs decide for the research activity from their own institutions, meaning that they review and issue approvals for these research activities. A centralized system of those IECs it is not yet available.
d) There is no procedural interaction between the national committees [(1), (2)] and the IECs (4) during the approval process of a bio-medical research application (including clinical research other than CDT, research with biological materials)/non bio-medical research application. The research application may be submitted in parallel to the national competent authority and to the institutional ethics committees.
References:
This report has been prepared by Ms. Loredana Dan, fellow of the Advanced Certificate Program in Research Ethics in Central and Eastern Europe, supported by the Fogarty International Center of the National Institutes of Health, Award Number R25TW007085.
-
Program supported by U.S. National Institutes of Health Research Grant R25 TW7085, funded by the Fogarty International Center, the National Institute of Environmental Health Sciences, the National Heart Lung and Blood Institute, and the National Institute on Drug Abuse.
Universities:
<- National Information
